Background
Recognizing the broad move toward open science, we’ve seen the following changes in expectations (outside NPS) since the inception of the I&M program.
Broad adoption of open-data and open-by-default practices.
A move in the scientific disciplines toward considering and publishing data sets as independently-citable scientific works.
Routine assignment of digital object identifiers (DOIs) to datasets to facilitate location, reuse, and citation of specific data
Increased transparency and reproducibility in the processing and analysis of data.
Establishment of peer reviewed “data journals” dedicated to publishing data sets and associated documentation designed to facilitate their reuse.
Expectation that science-based decisions are based on peer-reviewed, reproducible, and open science by default.
Data release reports are designed to parallel external peer-reviewed scientific journals dedicated to facilitate reuse of reproducible scientific data, in recognition that the primary reason IMD data are collected is to support science-based decisions.
Note that publication in a data release report series (not mandated) is distinct from requirements to document data collection, processing, and quality evaluation (mandated; see below). The establishment of a data release report series is intended to facilitate and encourage this type of reporting in a standard format, and in a manner commensurate with current scientific norms.
A template for creating data release reports to be published in the DRR series. We have also developed procedures for authoring DRR reports in Microsoft Word and porting them to the appropriate format.
Definitions
Reproducibility. The degree to which scientific information, modeling, and methods of analysis could be evaluated by an independent third party to arrive at the same, or substantially similar, conclusion as the original study or information, and that the scientific assessment can be repeated to obtain similar results (Plesser 2017). A study is reproducible if you can take the original data and the computer code used to analyze the data and reproduce all of the numerical findings from the study. This may initially sound like a trivial task but experience has shown that it’s not always easy to achieve this seemingly minimal standard (ASA 2017, Plesser 2017).
Transparency. Full disclosure of the methods used to obtain, process, analyze, and review scientific data and other information products, the availability of the data that went into and came out of the analysis, and the computer code used to conduct the analysis. Documenting this information is crucial to ensure reproducibility and requires, at minimum, the sharing of analytical data sets, relevant metadata, analytical code, and related software.
Fitness for Use. The utility of scientific information (in this case a dataset) for its intended users and its intended purposes. Agencies must review and communicate the fitness of a dataset for its intended purpose, and should provide the public sufficient documentation about each dataset to allow data users to determine the fitness of the data for the purpose for which third parties may consider using it.
Decisions. The type of decisions that must be based on publicly-available, reproducible, and peer-reviewed science has not been defined. At a minimum it includes any influential decisions, but it may also include any decisions subject to public review and comment.
Descriptive Reporting. The policies listed above are consistent in the requirement to provide documentation that describes the methods used to collect, process, and evaluate science products, including data. Note that this is distinct from (and in practice may significantly differ from) prescriptive documents such as protocols, procedures, and study plans. Descriptive reporting should cite or describe relevant science planning documents, methods used, deviations, and mitigations. In total, descriptive reporting provides a clear “line of sight” on precisely how data were collected, processed, and evaluated. Although deviations may warrant revisions to prescriptive documents, changes in prescriptive documents after the fact do not meet reproducibility and transparency requirements.
Policy Requirements
NPS Requirements
DO11B-a, DO 11B-b, OMB M-05-03 (Peer review and information quality):
Scientific information must be appropriately reviewed prior to use in decision-making, regulatory processes, or dissemination to the public, regardless of media.
As per OMB M-05-03 “scientific information” includes factual inputs, data, models, analyses, technical information, or scientific assessments related to such disciplines as the behavioral and social sciences, public health and medical sciences, life and earth sciences, engineering, or physical sciences.
Methods for producing information will be made transparent, to the maximum extent practicable, through accurate documentation, use of appropriate review, and verification of information quality.
OMB M-19-15 (Updates to Implementing the Information Quality Act):
Federal agencies must collect, use, and disseminate information that is fit for its intended purpose.
Agencies must conduct pre-dissemination review of quality [of scientific information] based on the likely use of that information. Quality encompasses utility, integrity, and objectivity, defined as follows: a) Utility – utility for its intended users and its intended purposes, b) Integrity – refers to security, and c) Objectivity – accurate, reliable, and unbiased as a matter of presentation and substance.
Agencies should provide the public with sufficient documentation about each dataset released to allow data users to determine the fitness of the data for the purpose for which third parties may consider using it. Potential users must be provided with sufficient information to understand… the data’s strengths, weaknesses, analytical limitations, security requirements, and processing options.
Reproducibility requirements for Influential Information. Note that because this may not be determined at the time of collection, processing, or dissemination this should be the default for NPS scientific activities:
Analyses must be disseminated with sufficient descriptions of data and methods to allow them to be reproduced by qualified third parties who may want to test the sensitivity of agency analyses. This is a higher standard than simply documenting the characteristics of the underlying data, which is required for all information.
Computer code used to process data should be made available to the public for further analysis. In the context of results generated, for example, a statistical model or machine augmented learning and decision support, reproducibility requires, at a minimum transparency about the specific methods, design parameters, equations or algorithms, parameters, and assumptions used.
Reports, data, and computer code used, developed, or cited in the analysis and reporting of findings must be made publicly available except where prohibited by law.
NPS Guidelines
NPS guidelines on Use of Scientific Information Multiple policy and guidance documents require the use of best available science in decision-making. Additional requirements include:
SO 3369 (Promoting Open Science):
- Defines “best available science” as publicly-available, reproducible, and peer reviewed. Requires that any decisions or scientific conclusions must prioritize the use of publicly-available, reproducible, and peer-reviewed science. Decisions or conclusions not based on such must include an explanation of why the alternative is the best available information. Effective as of 28 September 2018 with no transition period.
DO 11B (Ensuring Objectivity, Utility, and Integrity of Information Used and Disseminated by the National Park Service):
- The NPS will ensure that information it releases to the public or utilizes in management decisions will be developed from reliable data sources that provide the highest quality of information at each stage of information development.
I&M Requirements
NPS-75 (Inventory and Monitoring Guidelines):
An annual summary report documenting the condition of park resources should be developed as part of the annual revision of the parks Resource Management Plan.
An annual report provides a mechanism for reviewing and making recommendations for revisions in the [Protocol/SOPs].
[Inventory] data obtained should be archived in park records and, when appropriate, a report should be written summarizing findings.
Reporting requirements as per IMD directive
IMD Reporting and Analysis Guidance
- Annual Analyses Required of all Monitoring Protocols: Conduct an annual data review to address whether there is any unexpected variability or outliers, and whether any protocol changes or additional studies may be needed. Part of the review must assess the data against standards defined in the protocol narrative, data quality standards document or quality assurance plan, and document whether those standards were met. When data are not available for review during the year they are collected (for example, when data have been submitted to a lab for analysis), review must be conducted the year the data are available. For example, if water quality and quantity data are typically reviewed in October and lab results for water quality are not available until the following March, these data must be reviewed during the following October review period, if not before.
Implications
Because all of the data IMD collects is intended for use in supporting science-based decisions as per our program’s five goals, and is intended for use in planning (the decisions of which are subject to public comment as per NEPA requirements), this means that by default:
All analytical work we do should be reproducible to the extent possible. Analytical work includes both statistical analysis and reporting of data as well as quality control procedures where data are tested against quality standards and qualified or corrected as appropriate.
Full reproducibility may not be possible in all cases, particularly where analytical methods involve subject matter expertise to make informed judgments on how to proceed with analyses. In such cases, decisions should be documented to ensure transparency.
All IMD data should be published with supporting documentation to allow for reproduction of results.
All IMD data should be evaluated to determine whether they are suitable for their intended use.
All IMD data should be published with information fully describing how data were collected, processed, and evaluated.
All data should be published in open formats that support the FAIR principles (Findable, Accessible, Interoperable, and Resuable).
Scope
(for the NPS Inventory & Monitoring Program)
General Studies
Any project that involves the collection of scientific data for use in supporting decisions to be made by NPS personnel. General study data may or may not be collected based on documented or peer-reviewed study plans or defined quality standards, but are in most cases purpose-driven and resultant information should be evaluated for the suitability for—and prior to—their use in decision support. These data may be reused for secondary purposes including similar decisions at other locations or times and/or portions of general study data may be reused or contribute to other scientific work (observations from a deer browsing study may be contribute to an inventory or may be used as ancillary data to explain monitoring observations).
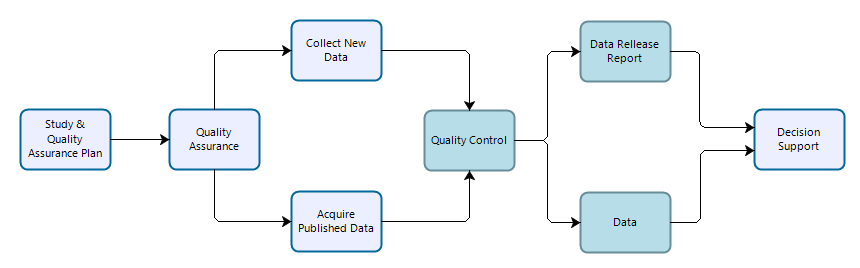
Figure 1: Workflow for data collection, processing, dissemination, and use for general studies. Teal-colored boxes are subject to reproducibility requirements.
Vital Signs Monitoring
Vital signs monitoring data are collected by IMD and park staff to address specific monitoring objectives following methods designed to ensure long-term comparability of data. Procedures are established to ensure that data quality standards are maintained in perpetuity. However, because monitoring data are collected over long periods of time in dynamic systems, the methods employed may differ from those prescribed in monitoring protocols, procedures, or sampling plans, and any deviations (and resultant mitigations to the data) must be documented. Data should be evaluated to ensure that they meet prescribed standards and are suitable for analyses designed to test whether monitoring objectives have been met. Monitoring data may be reused for secondary purposes including synthesis reports and condition assessments, and portions of monitoring data may contribute to inventories.
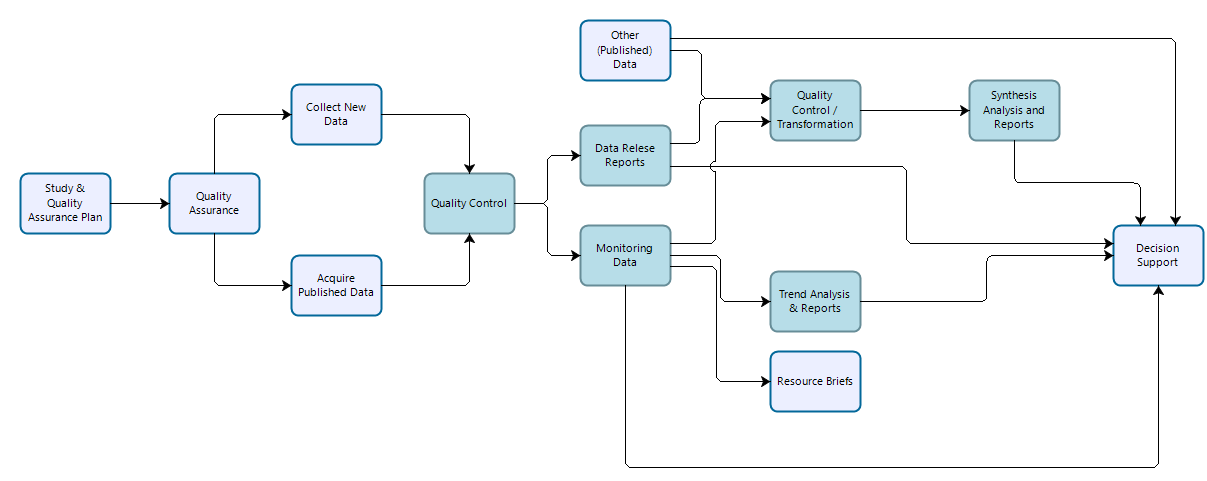
Figure 2: Workflow for data collection, processing, dissemination, and use for vital sign monitoring efforts. Teal-colored boxes are subject to reproducibility requirements.
Inventory Studies
Inventory study data are similar to general study data in that they are time- and area-specific efforts designed to answer specific management needs as well as broader inventory objectives outlined in project-specific study plans and inventory science plans. Inventory studies typically follow well-documented data collection methods or procedures, and resultant data should be evaluated for whether they are suitable for use in supporting study-specific and broader inventory-level objectives. Inventory study data are expected to be reused to meet broader inventory level goals, but may also support other scientific work and decision support.
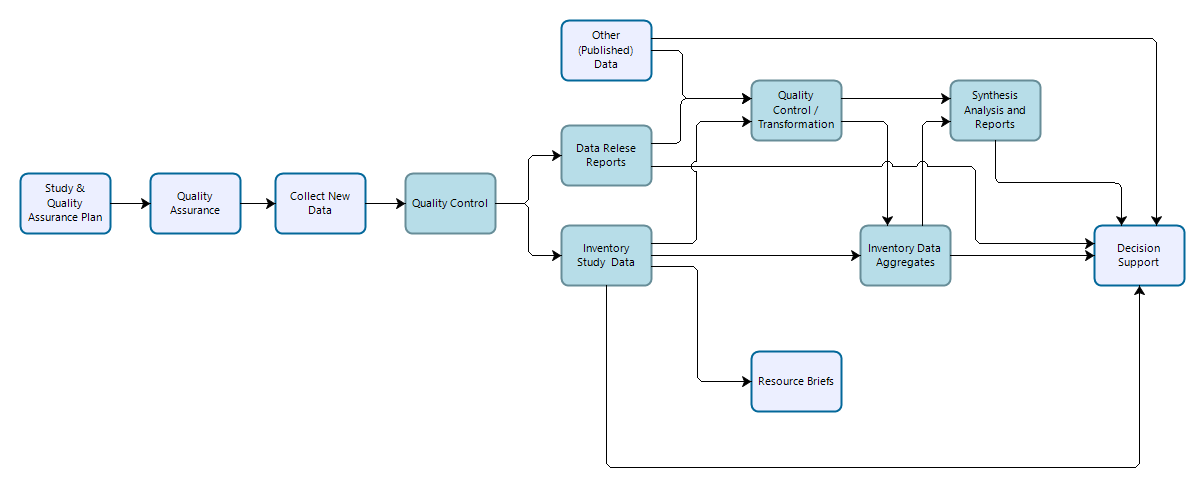
Figure 3: Workflow for data collection, processing, dissemination, and use for inventory studies. Teal-colored boxes are subject to reproducibility requirements.
American Statistical Association (ASA). 2017. Recommendations to funding agencies for supporting reproducible research. https://www.amstat.org/asa/files/pdfs/POL-ReproducibleResearchRecommendations.pdf.
Plesser, H. E. 2017. Reproducibility vs. Replicability: A brief history of a confused terminology. Front. Neuroinform. 11:76. https://doi.org/10.3389/fninf.2017.00076.